Impedance spectroscopy on Li-ion cells via reference electr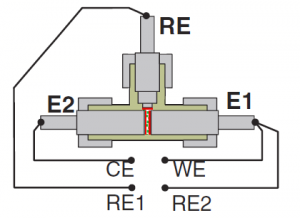 ode
ode
Three-electrode-setups consisting of a working electrode, a counter electrode and a reference electrode are well established in electrochemical investigations of experimental lithium-ion cells. The basic idea of such a three-electrode setup is providing a point with a fixed potential (the reference electrode), which is realized by inserting an electrode without any current flow. Relative to this fixed potential both electrode potentials are monitored and the resulting electrode overpotentials at a given current are determined.
However, when performing impedance spectroscopy on a three-electrode setup, the potential distribution within the cell changes with frequency (if the system does not exhibit perfect symmetry). This leads to severe distortions in the single electrode impedances. The question how the artifacts depend on cell parameters was assessed with frequency-domain FEM modeling of the actual cell geometry, leading to an understanding of the parameter dependencies. (see reference [6] at the publications page)
Measurement of effective conductivities and contact resistances of Li-ion cell electrodes
The effective conductivity of lithium-ion battery electrodes has a strong impact on their performance and usable capacity. There are numerous works, dealing with improving the
effective conductivity by (a) doping of the active material, (b) carbon coating of the active material particles, (c) adding carbon-based conductive additives and (d) compressing the electrodes.
A second parameter has an impact on the overall electrode resistance: the contact resistance between the electrode coating and the current collector. There is only one method which is capable to derive both parameters, the effective electronic conductivity and the contact resistance from a measurement on an electrode while attached to the current collector. However, it is rarely used and requires electrode samples that are much larger than the measured area (5-10 cm).
We have developed a new method to measure both, the effective conductivity and the contact resistance to the current collector of a dry porous electrode. It is based on a measuring the potential at the electrode surface as a function of the radius, while a dc current is induced at the center of the electrode disc, with the current collector connected to ground.
The only sample prepar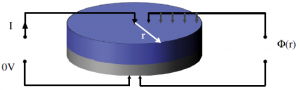 ation necessary is to cut out a disk-shaped electrode and to measure the thickness of the electrode coating. As the analysis of the measurement is based on numerical simulations (acounting for the sample and probe geometry), no simplifying assumptions have to be made. This allows also for using the identical electrode (18 mm diameter) for conductivity and electrochemical measurements. (see reference [12] at the publications page)
ation necessary is to cut out a disk-shaped electrode and to measure the thickness of the electrode coating. As the analysis of the measurement is based on numerical simulations (acounting for the sample and probe geometry), no simplifying assumptions have to be made. This allows also for using the identical electrode (18 mm diameter) for conductivity and electrochemical measurements. (see reference [12] at the publications page)
